The U.S. Food and Drug Administration (FDA) has issued a nationwide recall of prazosin hydrochloride, a common blood pressure medication, after detecting potentially cancer-causing impurities. The recall affects over 580,000 bottles distributed across the country by Teva Pharmaceuticals USA and Amerisource Health Services.
Why the Recall Happened
The recall was triggered when testing found traces of nitrosamines, a group of chemicals known to pose a cancer risk if people are exposed to high levels over time. Nitrosamines can form during the manufacturing or storage process of certain drugs, especially when not properly controlled.
While the presence of these impurities may not cause immediate health issues, long-term use of contaminated medication can increase the risk of developing cancer. The FDA classified this recall as a Class II risk, which means exposure could cause temporary or medically reversible health problems, but the chance of severe injury is lower.
What Is Prazosin and Who Uses It?
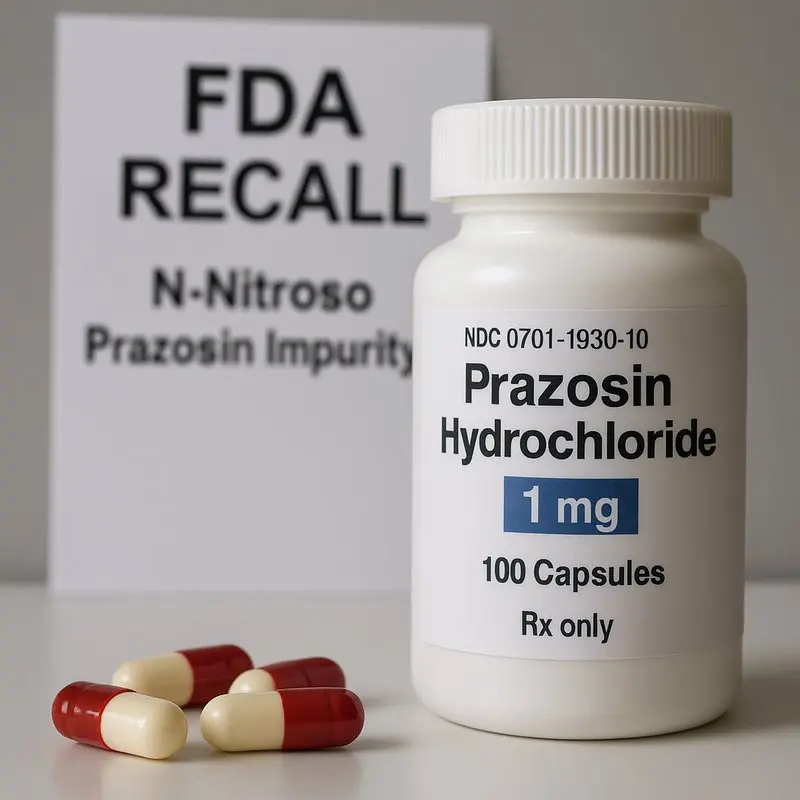
Prazosin hydrochloride is widely prescribed to treat high blood pressure (hypertension) by relaxing blood vessels, making it easier for blood to circulate. Interestingly, it’s also used to help people suffering from post-traumatic stress disorder (PTSD) by reducing nightmares and improving sleep quality.
Despite its benefits, the discovery of contamination prompted the manufacturers to act quickly, issuing a voluntary recall to protect patient safety.
Comparison: Prazosin vs. Other Blood Pressure Medicines
| Feature | Prazosin Hydrochloride | Lisinopril / Amlodipine |
|---|---|---|
| Main Function | Relaxes blood vessels to lower BP | Lowers BP by reducing heart strain or relaxing arteries |
| Extra Uses | PTSD-related nightmares and sleep aid | Focused on heart and blood pressure only |
| Recall Reason | Nitrosamine contamination | Varies — often due to labeling or dosage issues |
| FDA Risk Level | Class II | Depends on issue severity |
What Patients Should Do
If you currently take prazosin, don’t panic — not all lots are affected. It’s important to check your medication label for the manufacturer and batch number. If you’re uncertain, contact your pharmacy or doctor for clarification.
Do not stop taking your medication abruptly unless your healthcare provider instructs you to. Stopping blood pressure medicine suddenly can cause dangerous spikes in blood pressure or other side effects. Your doctor can help you switch to an unaffected batch or a suitable alternative safely.
FDA’s Role and Future Steps
The FDA continues to strengthen its oversight of manufacturing practices to prevent nitrosamine formation in medications. It’s also working closely with drug makers to improve testing and storage protocols, ensuring that patients receive only safe and effective treatments.
Final Thoughts
The blood pressure drug recall involving prazosin hydrochloride highlights how important quality control and safety monitoring are in the pharmaceutical industry. While recalls can create anxiety, they also show the system’s commitment to protecting public health. Patients should remain calm, stay informed, and seek guidance from trusted healthcare professionals before making any medication changes.


